Each cell has a phenome & is an independent functioning life.
The phenome is the sum of many functions, or collective life, for each cell.
The phenome is the set of all phenotypic traits expressed by a cell or organism, central to all questions in biology, in which the sum of the parts creates a whole.
Each cell has a phenome & is an independent functioning life.
The phenome is the sum of many functions, or collective life, for each cell.

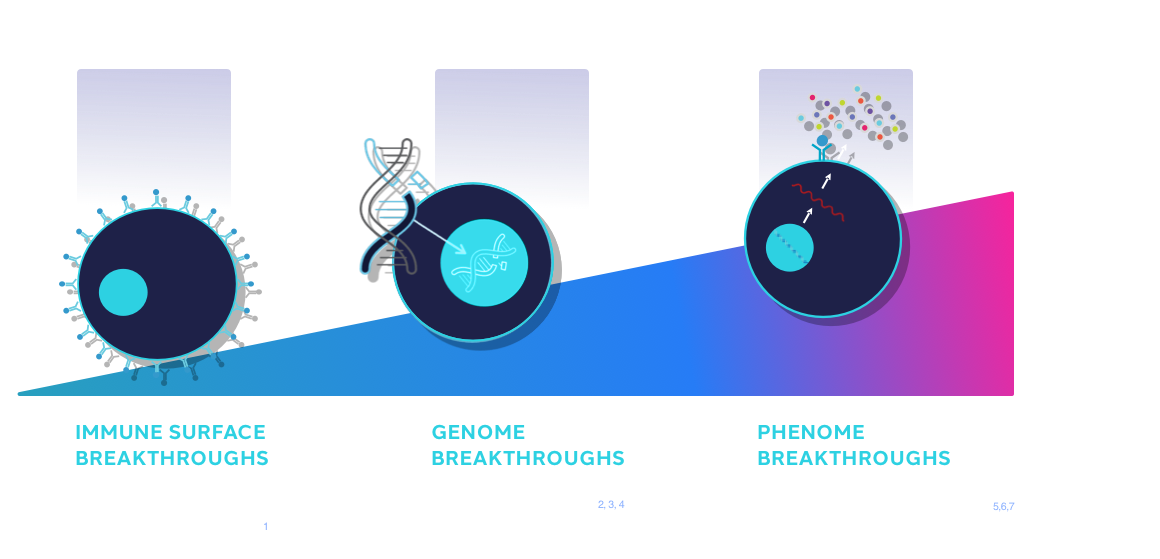
The evolution of functional biology began with immune surface breakthroughs and the advent of flow cytometry, which defined CD4 T cells and enhanced the ability to combat infectious diseases. Following this, significant genome breakthroughs led to the ability to change T cell receptors and create novel therapies such as CAR-T. The era of phenome is now upon us as we increase our scope of biological understanding, enabling the engineering of new therapies with optimal functional phenomes.
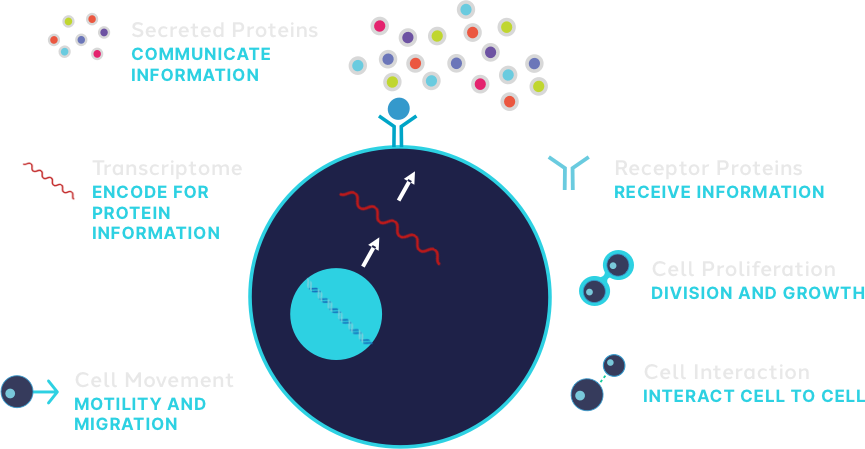
In particular, today's solutions typically look at three things:
Our technologies uniquely capture aspects of the phenome required to accelerate biological solutions for thousands of labs in this next era of the phenome.
The Bruker Cellular Analysis Suite of discovery to clinical research technologies can capture the phenome, the collective sum of the parts of the cells, the phenotype, and the functional phenotype, and leverage that to pick the best whole cells and pick the best patient response.
Bruker Cellular Analysis uniquely reveals Potent and Dysfunctional Cells — highly polyfunctional cells that are early actors and orchestrators in the immune response. These rare subsets of highly functional cells can be beneficial (Potent Cells) or detrimental (Dysfunctional Cells). Detecting these cells is critical for developing more effective immune therapies and vaccines.
Potent cells are rare subsets of highly-polyfunctional cells that orchestrate the response against tumors and correlate to potency, persistence, and survival.
Dysfunctional cells are highly-polyfunctional cells that drive disease progression and correlate to inflammation, toxicity, and resistance.
The proteome is complex and requires direct detection. Dynamic in vivo biology is the captured by the proteome— the key to advancing medicines and diagnostics.
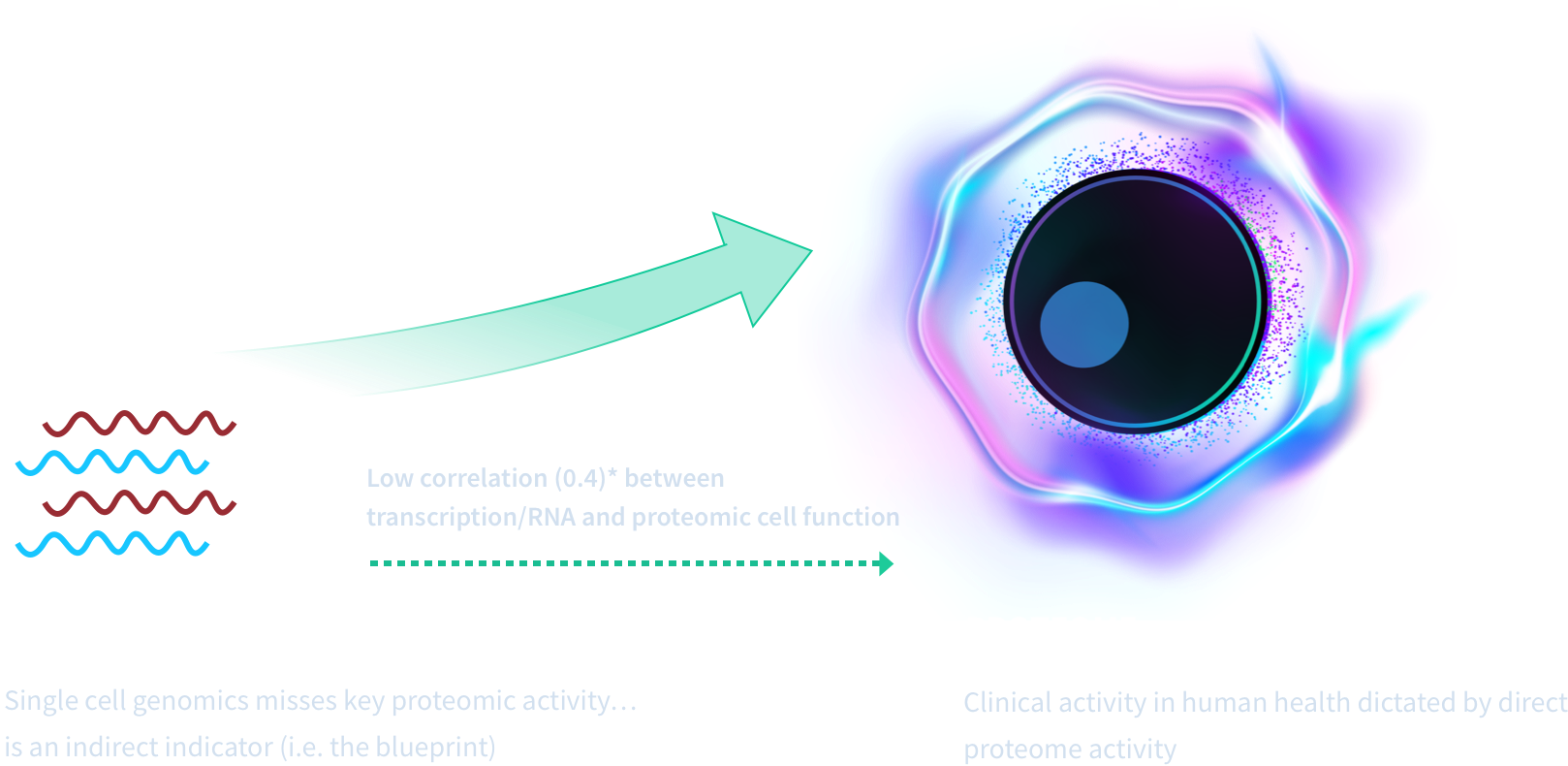
Bruker Cellular Analysis technology can uniquely reveal highly functional cells — the 5-10% of cells that are early actors of an immune response. We achieve this by detecting each cell's highly multiplexing cytokines, chemokines, and phosphoproteins.
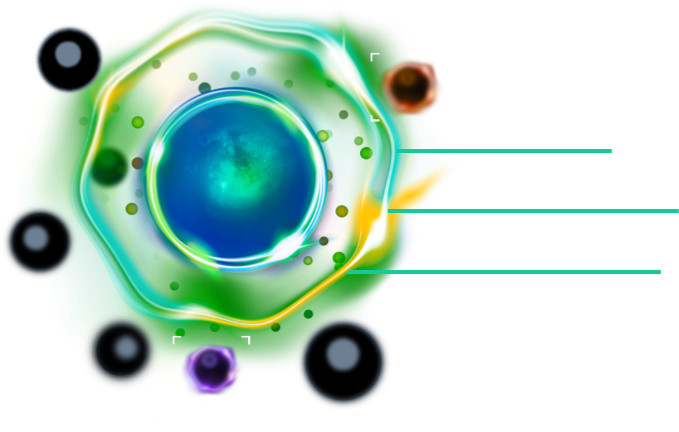
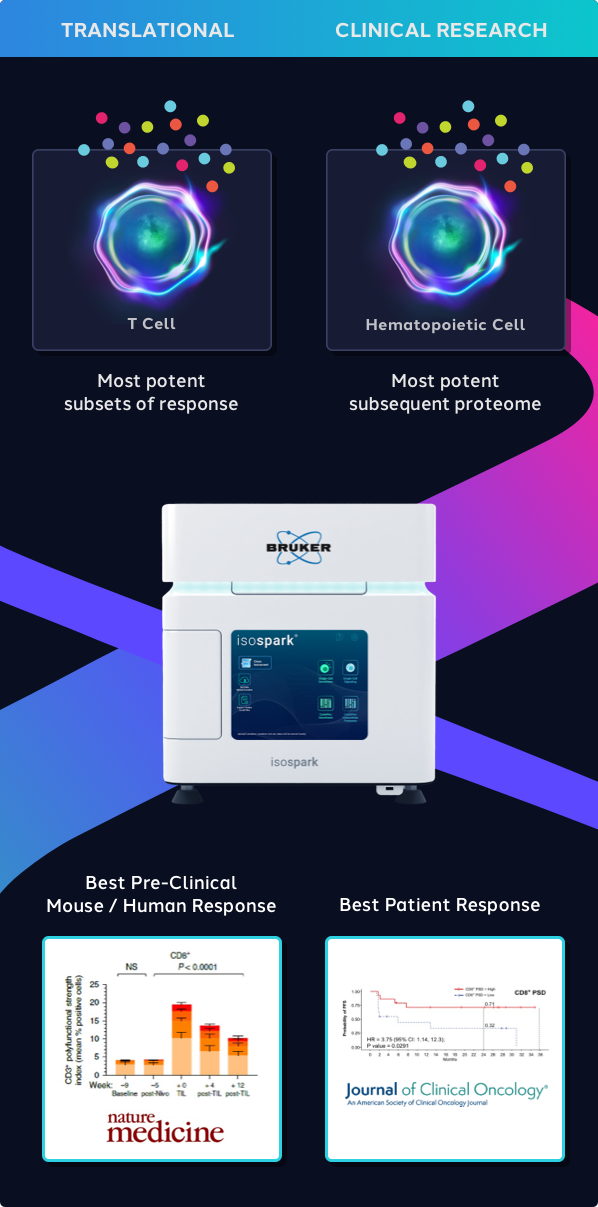
The IsoSpark's leap in innovation allows researchers to immediately detect the most potent immune cells and leverage that response data for preclinical and translational/clinical research.
The Beacon's breakthrough single-cell functional phenotyping tells you exactly which immune/production cell functionally produces key antibodies/proteins and the receptor/clone data, with the ability to retrieve that live cell.
This leap in innovation allows researchers to immediately detect the most potent cell and leverage it for discovery and bioprocessing.
Cytokine function dictates the response of each cell to the tumor:
With the leaders from Kite Pharma and NCI: in a 20-patient non-Hodgkin Lymphoma CAR-T study, the frequency of highly functional cells (~20-25%), i.e., High PSI of each CAR-T cell therapy product, predicted response - Flow Cytometry & Bulk ELISA did not. Functional single-cell biology uniquely provides this information and can be targeted to create better products before release.

Emergency Use Authorization of AstraZeneca’s Evuseld: Researchers at Vanderbilt used our Optofluidic platform to discover the antibodies from primary human tissues. Patients given a single injection of the antibody treatment were 83% less likely to develop symptomatic cases of the coronavirus than participants who were given a placebo.
In a separate trial, patients with mild-to-moderate COVID-19 who were given one dose of antibody treatment within three days of developing symptoms had a reduced risk of developing severe disease by 88%.
Results published in Nature Medicine.
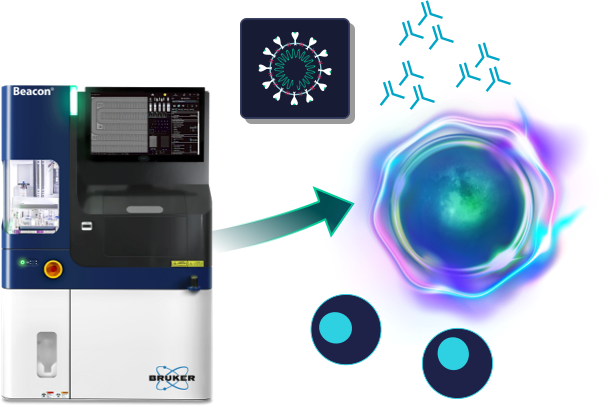
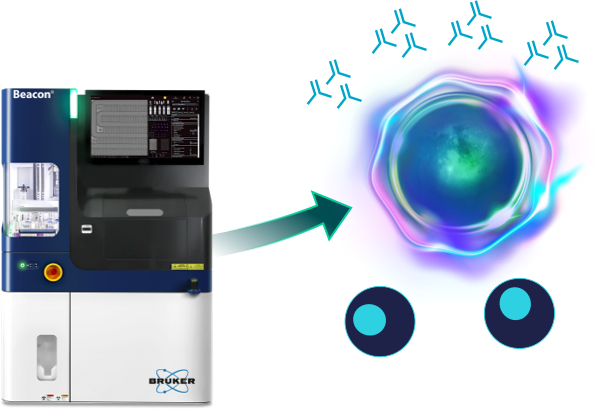
Every B cell can produce a unique antibody, and discovering the most potent antibody often requires finding the rate B cell in a highly diverse cell population.
Antibody discovery using the traditional hybridoma approach requires the immortalization of B cells by cell fusion that results in the death of >90% of B cells. This significantly reduces the chances of discovering novel antibodies against difficult targets.
Opto B Discovery workflows on the Beacon systems increase the probability of finding the best antibody by direct functional assessment of antibody products by single B cells.
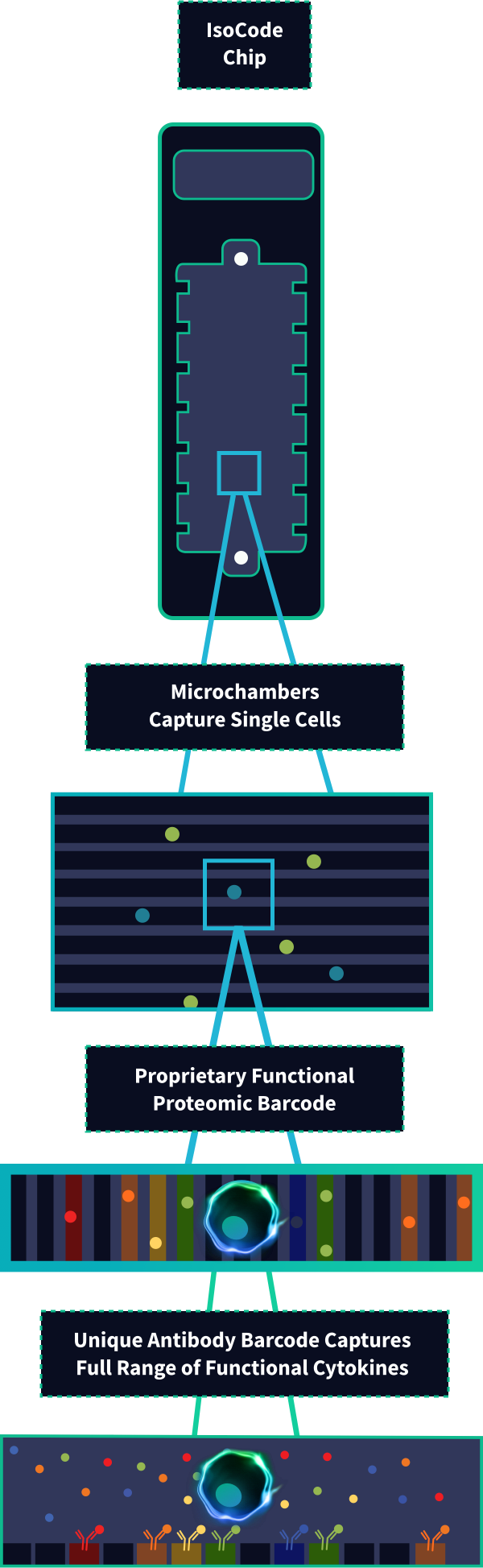
Our proprietary & patented “Proteomic Barcoded” IsoCode Chip, named the Scientist's number #1 innovation, works by detecting 30+ cytokines per cell, measuring the functional phenotype of each immune cell.
This breakthrough technical innovation allows for complete single-cell functional characterization — something that was not possible before.
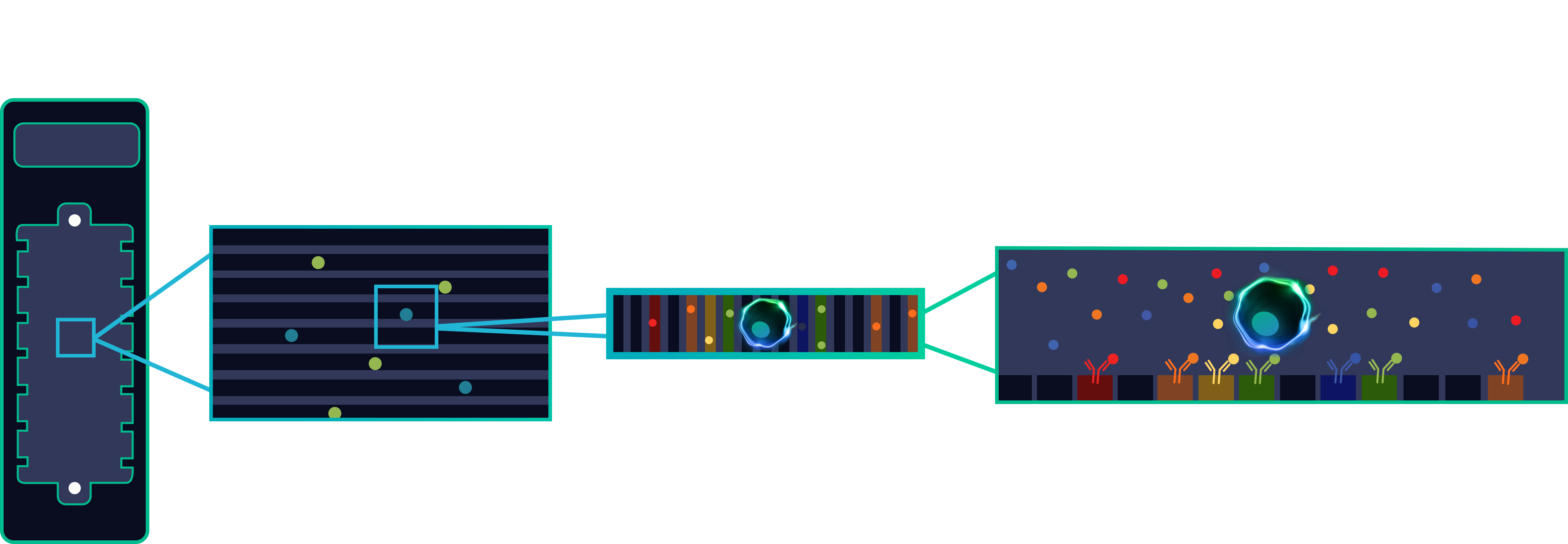
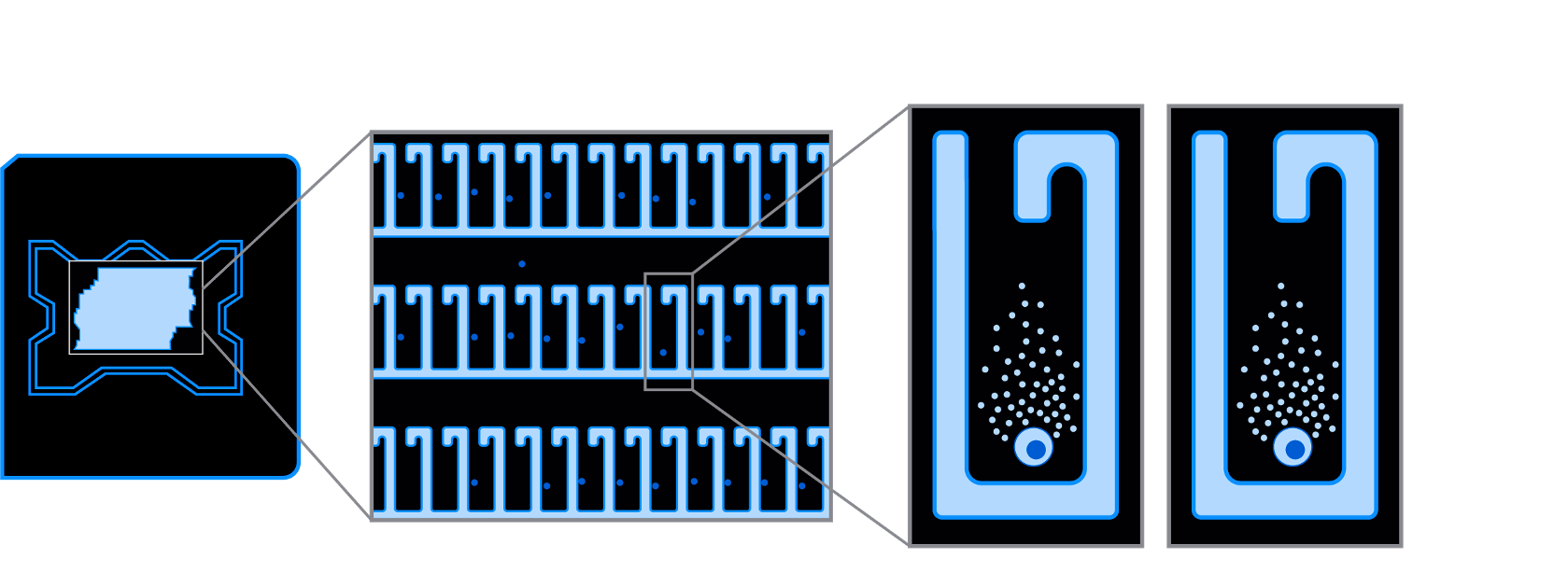
Our OEP fluidics uses light and millions of light-actuated pixels to move individual cells to be isolated, cultured, assayed, and exported. Each cell or clone is imaged and monitored in real-time in a NanoPen™ chamber on our OptoSelect™ chips to provide rich visual data early and often. Our Optofluidic product suite delivers cell processing and deep profiling with more information about cell function than any other technology.
Gain deeper access to in vivo biology through highly-multiplexed functional cell biology.
Instantly analyze, dissect, visualize, and export your data with a click of a button — no coding required.
Fully automate your workflow with our all-in-one, intuitive system that fits on your lab bench.